III. Reaction Methodology Development
III.1. α-functionalization of Amides
Amide is one of the most important motif in biological systems as well as in pharmaceutical industry. Amide is also common intermediates in chemical synthesis of many complex molecules. We are exploring efficient methodology for α-functionalization of amide with different functional groups to expand the scope of amide molecules in medicinal chemistry.
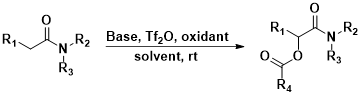
Scheme III.1: Synthetic scheme of α-esterification of protected amides
1. Khan, M.I.H.; Yang, J.; Kim, S.J.; Le, H.V.* A New Reaction of Togni Reagent II: α-C–H Ester-Functionalization of Tertiary Amides. Medicinal Chemistry Research 2025
III.2. Water mediated Isooxazole ring formation
Isoxazoles, five-membered heterocycles, are found in numerous bioactive natural products and synthetic molecule drugs. Therefore, new method to develop efficient and green route to synthesize isoxazoles is always desirable to increase the scope of this heterocycle in medicinal chemistry. We synthesized 3,4,5-trisubstituted isoxazoles in water via a [3+2]-cycloaddition of nitrile oxides and 1,3-diketones, β-ketoesters, or β-ketoamides.

Scheme III.2: Synthetic scheme of water-mediated 3,4,5-trisubstituted isoxazoles.
1. Hossain, M.I.; Khan, M.I.H.; Kim, S.J.; Le, H.V.* Synthesis of 3, 4, 5-Trisubstituted Isoxazoles in Water via a [3+ 2] Cycloaddition of Nitrile Oxides and 1, 3-Diketones, β-Ketoesters, or β-Ketoamides: Base-mediated and Keto-enol-controlled Mechanism. Beilstein Journal of Organic Chemistry 2022, 18, 446–458.